Use of a coagulant system developed at Lincoln University on dairy effluent has been shown to overcome the risks of phosphorus leaching in light soils. Anne Lee reports.
Leaching of phosphorus from light, stony soils on effluent areas has only recently been highlighted as a problem but Lincoln University PhD student Chris Chisolm has already found a way to combat the issue – albeit with some investment into a new effluent system.
Chris is shearing through his summer break – between completing his honours degree and starting his PhD – and although he’s got his sights on cracking the 500-a-day tally in the short term, long-term his mind is firmly in the science of the soil. Testament to his skill in his scientific endeavours Chris’ honours project for his Bachelor of Agricultural Science was published in September in the New Zealand Journal of Agricultural Research.
His studies found the chemical process used to separate the solid fraction of farm dairy effluent from water in the ClearTech effluent system can all but halt phosphorus loss when the effluent and/or the clarified water is applied to the soil.
And that’s turned out to be an extremely important fact given a study published only last year found that, based on 14 years of data from Lincoln University Dairy Farm (LUDF), phosphorus is leaching at a much greater rate through light, stony soils on effluent areas than previously understood. (See sidebar below for more.)
Science of the floc
The ClearTech system was developed by Lincoln University soil science professors Keith Cameron and Hong Di in collaboration with commercial partner Ravensdown. The science lies in the chemical reactions that occur when a commonly used coagulant, ferric sulphate, Fe2(SO4)3 often used in municipal drinking water treatment, is mixed with the raw farm dairy effluent.
The coagulant sets up a sweep floc motion as it allows the colloids or solid particles in the effluent to come together.
That sweep floc motion causes the combined colloids to swirl and sweep down and around until they settle at the bottom of the solution, leaving clarified water at the top. But it’s not just clear water, it’s clean too with almost all of the nasty bacteria gone.
E. coli bacteria are both trapped between the colloid particles that have been bound together and killed through a weakening of the bacteria’s cell walls. At the same time a chemical reaction brings the phosphorus (from the effluent) and iron (in the coagulant) together to form more stable iron phosphate compounds.
What’s happening underground
Chris’ honours study used lysimeters representing a light, stony dairy pasture and looked at the effects of applying:
- the clarified water on its own,
- untreated dairy effluent,
- treated effluent (solid portion from ClearTech process),
- a combination of clarified water and treated effluent,
- clarified water spiked with ammonium chloride (to simulate clarified water repeatedly recycled and used as yard ashdown water),
- fresh water – as a control.
He measured the amount of E. coli, nitrogen, iron and phosphorus in the leachate and also looked at greenhouse gas emissions and pasture growth.
His results not only confirmed previous studies, it also found new information too as the effects of clarified water applications to soil hadn’t been closely studied before.
No dissolved reactive phosphorus (DRP) was detected in leachate at all following the application of clarified water, nor was it detected after the spiked clarified water was applied. DRP was 99.5% lower in leachate after treated effluent was applied compared with untreated effluent. (Table one.)
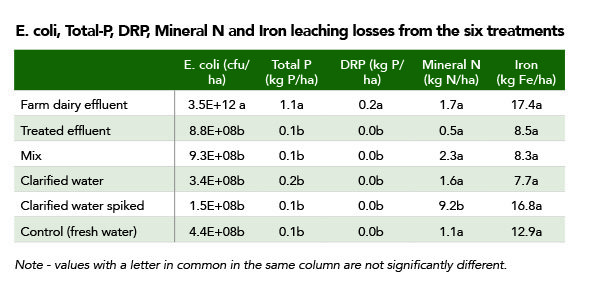
Total phosphorus losses were also drastically cut with no significant difference in leachate levels when compared with applying clean water to the lysimeters.E. coli levels from treated effluent, clarified water and fresh water were all close to zero – a 99.9%+ reduction compared with untreated effluent.
Nitrogen losses from all treatments were relatively low with no significant difference between untreated effluent apart from the spiked clarified water which had additional nitrogen from the added ammonium chloride. Even then, Chris says the losses of mineral nitrogen were equivalent to less than 10kg N/ha which is relatively small compared with losses on grazed dairy pastures that mostly come from cow urine.
Iron losses were not significantly different to losses detected in the control or from lysimeters where untreated effluent was applied.
Extra modelling to check any effect on iron levels in soil from applying the treated effluent showed that any extra iron going into the soil from the coagulant was so minor compared with levels already in soil that even over a 10-year period of application it’s unlikely any excessive build-up of iron or leaching will occur.
Chris also found no significant difference in greenhouse gas emissions or in pasture production. Other major benefits of the system are savings on water and effluent storage because the clarified water removed from the effluent can be recycled and used in yard washdown.
LUDF has been using the system this season and expects to save 6 million litres of water.
Light soil risks with phosphorus
Despite following all best practice guidelines phosphorus is at risk of leaching down through light stony soils on effluent areas.
AgResearch scientist Richard McDowell’s study published in the Agriculture, Ecosystems and Environment journal last year used information from 14 years of leachate data collected on the Lincoln University Dairy Farm (LUDF).
He found from 2001 to 2015 the average total phosphorus loss from the free-draining, shallow Eyre soils at LUDF, where farm dairy effluent had been applied, was six times more than other areas.
The analyses measured the loss at 1.46kg P/ha/year – significantly more than from the non-effluent area, also on the Eyre soil, where an average of 0.25kg P/ha/year was measured in leachate.
The same hasn’t happened on the moderately drained, deeper Templeton soils though.
There the average loss was 0.12kg P/ha/year for both effluent and non-effluent areas.
The LUDF applies its effluent via sprinklers on an underslung line on its pivot irrigator.
The team follows industry and regional council rules in applying it – no more than 10mm of effluent applied at any one time, not applying it within 24 hours of soil saturation, no ponding and ensuring applications keep soil test phosphorus concentrations at or below agronomic optimum to meet pasture demand.
Despite this, Richard says, over time phosphorus was leaching through the soil profile on the light soil likely because of the soil’s free-draining properties and macropores combined with possible saturation of the macropore walls making phosphorus less held by those walls.






